Menopause Study Results
The Use of a Vaginal Moisturizer Containing Vitamin B3 (Vibrel™ for Women) in Perimenopausal and Menopausal
Women Complaining of Sexual Dysfunction: Results of a Pilot Study.
John A. Sunyecz, M.D., Kelly Skovira, D.N.P. -
MenopauseRx, Inc. Uniontown, PA, USA
Introduction: ^ Return to top
-

Download Poster (335 KB - PDF)
|
Sexual difficulties are very common during the perimenopause and menopause transition.
- Data from the National Health and Social Life Survey indicate that nearly one half of
US women, including those at midlife, have a problem in one or more aspects of sexual functioning.
- Early to late menopausal transition, the percentage of women with sexual dysfunction
increased from 42% to 88%1.
- Declining estradiol levels during menopause can interfere with sexual function due to
numerous changes. Genital effects of estrogen include vaginal pliability, lubrication,
and local pleasure sensation in the sexual arousal phase. Hypoestrogenism causes
changes in the four layers of the vaginal wall that may result in dyspareunia and a loss in the quality of the genital arousal response2.
- A patent pending trans-dermal delivery system has been developed that allows a vitamin B3 based vaginal moisturizer to act as a vasodilator. This vasodilation is thought to increase blood flow to the genital area and assist in the process of natural lubrication.
Objective: ^ Return to top
To examine the use of a vaginal moisturizer containing vitamin B3 (Vibrel™ for Women)
in perimenopausal and menopausal women with sexual dysfunction.
Methods: ^ Return to top
- Study subjects were recruited from the MenopauseRx, Inc. database. This opt-in database
consists of women generally between the ages of 35 and 64 years.
- An e-newsletter was created and sent to the database requesting participants who
were experiencing sexual problems during the menopause/perimenopause transition.
- One of the authors (K.S.) contacted potential participants who were evaluated for study
inclusion based upon defined inclusion and exclusion criteria.
- Inclusion criteria included sexually active women over the age of 45 who were experiencing sexual difficulties.
- Exclusion criteria included women with cardiac, thyroid, or psychiatric conditions
(including the use of SSRI and/or other antidepressant medications), a history of sexualabuse, or if they regularly consumed more than two standard alcoholic drinks per day.
- Eligible women were invited to participate in the study and a consent form was sent.
- Upon electronic signature of the consent form, the participants were directed to an
online survey utilizing the Female Sexual Functioning Index (FSFI) and Sexual Quality
of Life Questionnaire – Female (SQOL-F).
- Upon completion of this baseline survey, the participants were sent study materials, including study sample and directions.
- The participants were asked to stop using any other vaginal lubricants and/or moisturizing agents for the duration of the study.
- Directions were to use the study vaginal moisturizer with every sexual act. They were
instructed to apply a small amount of moisturizer (about the size of a pea) to the clitoris, labia and vagina 1-15 minutes before each sexual encounter.
- The participants were asked to fill out the FSFI after the fourth week of the study. They were also asked to complete the FSFI and SQOL-F at the end of the study (8th week).
- Study participants were not paid for enrollment in the study.
Study Population: ^ Return to top
- The study population was derived exclusively from the MenopauseRx, Inc. database.
- Within 48 hours of sending the recruitment e-newsletter, 538 women initially responded and expressed interest in this pilot study.
- Of the first 108 women, 64 women between the ages of 45 and 60 met inclusion criteria and were enrolled and study recruitment was closed.
- Three women did not respond to the consent after initially expressing interest, while two women opted out of the study during the consent process.
- Fifty nine women agreed to the consent.
- Four women opted out of the study before completing the baseline survey.
- Nine women opted out before completing the four week survey, while one participant opted out before completing the eight week survey.
- A total of 45 women (average age: 50.3 years) completed the eight week study.
Treatment: ^ Return to top
- The study vaginal moisturizer (Vibrel™ for Women) contained vitamin B-3 (niacin) which utilizes an innovative patent pending transdermal delivery system.
- The study sample utilized was an open label container.
- Each participant was supplied with three study vials (0.10 ounce/3.0 gram per vial).
Clinical Measurements: ^ Return to top
- The FSFI is a brief multidimensional scale for assessing sexual function in women. It was developed for the specific purpose of assessing domains of sexual functioning (e.g. sexual arousal, orgasm, satisfaction, pain) in clinical trials.
- The FSFI was validated in two groups of women, including subjects with sexual arousal disorder (determined by history) and age-matched controls.
- The instrument sensitively and reliably differentiates these two groups on all domains of sexual functioning.
- The FSFI has been validated to be useful for evaluation of treatment outcomes in a clinical trial situation.
- The Sexual Quality of Life-Female (SQOL-F) questionnaire was developed to assess the impact of female sexual dysfunction (FSD) on a woman's sexual quality of life.
- A SQOL-F item was originated through interviews with 82 women and addresses three concepts: impact on self-esteem, emotional well-being, and relationship impact.
- The SQOL-F showed good psychometric properties: convergent validity, discriminate validity, and test-retest reliability.
- The SQOL-F is a valid instrument for assessing the impact of FSD on quality of life and as an adjunct in evaluating FSD in clinical trials.
Statistical Analysis: ^ Return to top
- For the FSFI, statistical analysis was performed by calculating the average difference, standard error and level of significance from baseline to week 4, baseline to week 8 and week 4 to week 8 for each question.
- For the SQOL-F, the average difference, standard error and level of significance was calculated from baseline to week 8 for each question. Women who did not complete all of the surveys were not included in this report.
Results: ^ Return to top
- Patient Characteristics:
- The average age of participants who completed the 8 week study was 50.3 (range 45-60).
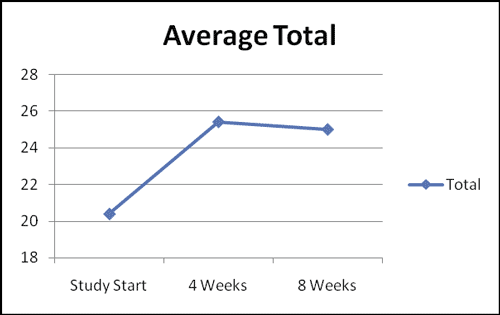
- The average FSFI score at baseline was 20.40 (+/- 2.20).
- The average FSFI score at the end of the study was 25.01 (+/- 2.20).
- The overall mean difference in FSFI scores from baseline to the end of the study was 4.60 (+/- 2.14; p-value < 0.01).
- The overall SQOL average difference was 0.68 with a standard error of 0.164, and a pvalue = 1%.
- Efficacy Outcomes:
- The FSFI contains questions related to a various domains of sexual functioning. These domains are broken down into 1) sexual desire or interest, 2) sexual arousal, 3) lubrication, 4) orgasms, and 5) satisfaction.
- These results are graphically represented below:
Average Total of the FSFI
Overall comparison between Six areas of the FSFI*
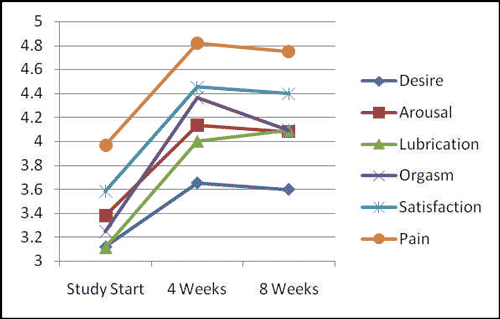
*Comparisons are not standardized. “Desire” is on a scale of (1.2, 6);
the remaining areas are on a scale of (0,6).
- Efficacy Outcomes Continued:
- The individual questions within the FSFI all revealed a positive impact after the eight week
trial.
- The most significant improvements were seen with the following questions (in descending
order):
- Over the past 4 weeks, how difficult was it to maintain your lubrication until completion of sexual activity or intercourse?
- Over the past 4 weeks, how often did you maintain your lubrication until completion of sexual activity or intercourse?
- Over the past 4 weeks, how satisfied have you been with the amount of emotional
closeness during sexual activity between you and your partner?
- Over the past 4 weeks, how satisfied were you with your ability to reach orgasm during sexual activity or intercourse?
- Over the past 4 weeks, how satisfied have you been with your overall sexual life?
- Over the past 4 weeks, how often did you become lubricated during sexual activity or
intercourse?
- Holding all other variables constant, the probability of the change from the beginning of the study until the end being due to chance alone is less 1%.
- Therefore, using the FSFI as a validated tool of sexual function, treatment with the study
vaginal moisturizer may have resulted in: improved sexual desire, arousal, lubrication, orgasms
and sexual satisfaction. The probability of this being due to chance alone was less
1%.
- Using the SQOL-F as a validated tool of sexual function, the most notable improvements
seen over the course of the study were with the following statements:
- worry about sexual life, including feeling less like a woman
- losing confidence as a sexual partner
- frustration with sexual life
- satisfaction with sexual life
- pleasure in sexual activity
- Each of these statements showed improvement over the course of the study. The
probability of this being due to chance alone was less than 1%.
- There were no study participants who dropped out of the study due to adverse reactions
and no adverse events were reported upon completion of the study.
Study Summary:
This pilot study revealed a baseline level of sexual dysfunction and
its negative impact on self-esteem, emotional well-being and impact
on relationships in the study population. After using the vaginal
moisturizer containing vitamin B3 ( Vibrel™ for Women) for eight
weeks, results demonstrated the probability that Vibrel™ for Women
may improve overall sexual functioning in women during the menopause
transition. An improvement in sexual quality of life was also noted during this pilot study.
As a result of this pilot trial, additional studies are warranted to further
elucidate these findings in a randomized, placebo controlled
trial.
At the present time, the results from this trial indicate that Vibrel™ is
a well tolerated, advantageous moisturizer and regular use may improve
sexual function in women with sexual dysfunction and improve
their sexual quality of life.
(1) Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition.
Fertil Steril. 2002 Apr; 77 Suppl 4:S42-8.
(2) Lara LA, Useche B, Ferriani RA, Reis RM, de Sá MF, de Freitas MM, Rosa e Silva JC, Rosa e Silva AC. The effects of
hypoestrogenism on the vaginal wall: interference with the normal sexual response. J Sex Med. 2009 Jan;6(1):30-9.
|





